Among all the procedures the most demanded
procedures is of column
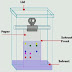
chromatography. In this procedure an absorbent is filled in a
one third glass tube with alumina or silica gel. Then the material is soaked in
selective solvent. Then the column is filled with slurry of absorbent and
solvent. An important thing to be kept in mind is that there should be no space
in the column. This type of packed column is termed as well packed column. This
method is a little different from the other methods as in this method a less
polar column is removed first. The reason for removing this element first is
that lesser the polar compound will be lesser it will be absorbed in the
stationary phase and in this way more polar compound can be extracted out
easily.
 chromatography is considered as an ideal
technique for separation whether it is any material solid or liquid. There is
another technique used in this column chromatography that is flash column
chromatography. This procedure is carried out with a mixture of two or more
solvents. This procedure can be adopted in both polar as well as non-polar
compounds. Some of the important things to be considered while separating
solvents from this procedure is that dichloromethane dissolve compounds in a
better way but at the same time silica will take longer time. Benzene is quite useful
but in case of non-polar component it shows its best efficiency. It is
sometimes even avoided because of its toxicity. If your compound is acidic
sensitive you can add 1% to 3% of triethylamine in the mixture in order to
neutralize the acidic effect in silica gel.
chromatography is considered as an ideal
technique for separation whether it is any material solid or liquid. There is
another technique used in this column chromatography that is flash column
chromatography. This procedure is carried out with a mixture of two or more
solvents. This procedure can be adopted in both polar as well as non-polar
compounds. Some of the important things to be considered while separating
solvents from this procedure is that dichloromethane dissolve compounds in a
better way but at the same time silica will take longer time. Benzene is quite useful
but in case of non-polar component it shows its best efficiency. It is
sometimes even avoided because of its toxicity. If your compound is acidic
sensitive you can add 1% to 3% of triethylamine in the mixture in order to
neutralize the acidic effect in silica gel. 











0 comments:
Post a Comment