Modern technology has yielded many different methods to help
process everyday compounds and mixtures in our lives. It starts out as a
complicated process but often yields to very common applications that you use
every day. Chromatography is a similar technological process, which in its
various types finds its application in both industrial and household levels.
What is
chromatography?
It is a technique used for separating or identifying
(sometimes both) the various components that make up a mixture. The process
uses a simple principle that each component of the mixture has various
tendencies of absorption or dissolution in to various surfaces and solvents
respectively. In the technique there is always a stationary phase (usually a thin layer of solid. Inert or
otherwise) through which a component (mobile
phase) is passed to separate the elements.
Different types of
chromatography:
1. Ion exchange
chromatography
This method is very similar to the process of partition
chromatography and uses a coated solid as the stationary phase. The liquid that
is passed through the layer is called the mobile phase and the process works on
the principle of opposite ions attracting each other. Domestic water softeners
and the water softeners attached with commercial dishwashers work on this
principle.
2. Paper chromatography:
This is the most common form of chromatography used in
everyday household to separate a mixture of substances. Different types of
paper here form the solvent. Separating inks from fountain pens and balls pens,
also separating food colorings or dyes are common examples of this type of
process. Thin layer
Chromatography is another variation on this process. However, the solvent
used here is a thin layer of solid such as aluminum supported on a base (inert),
which does not participate in the process. TLC finds industrial application in
the determination of the progress of a reaction by identifying and studying the
nature of each of the components.
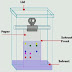
3. Liquid Column
Chromatography:
Liquid Column chromatography uses a liquid mobile phase
where the liquid is passed through an inert solid (e.g. Silica gel, cellulose) supported
in a column of glass. This acts as a stationary phase for the reaction. Flash chromatography is an alternative
technique, which works on the same principle as the liquid chromatography.
However, under the flash technique the solvent is forced to pass through the
column at a much higher pace. To achieve this, an inert gas like Nitrogen is
used to apply extra pressure on the solvent without reacting to it.
HPLC or High Performance Liquid Chromatography:
Today a much-evolved variation of liquid chromatography
finds its application in our industries. This new technique is called HPLC or
high performance liquid chromatography. In this case, the particles in the
stationary phase are made much smaller to help react with the mobile phase much
giving us much more accurate readings. This technique is used in a variety of
niche industries such as pharmaceutical, environmental and forensics. The
technique helps separate different trace components and then identify them
against known traces in the records. It is a very definitive analytical tool
for the pharmaceutical industries to understand the various concentrations of
medicinal drugs and study their interactions at a testing level.












0 comments:
Post a Comment