Chromatography in today’s world has
taken completely new dimension. With the advent of new technologies, new
methods are being adopted for separating compounds from any substance, whether
it is in liquid or solid form. But in case of flash chromatography, the process
is usually done with the mixture of two solvents, where one is polar while the
other one is non-polar. This method is also called as the state of art. In this
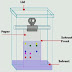
method the solvent is pressurized down the column with the help of external air
pressure. This will isolate the desired organic compound from the solvent. The
solvent choose to be used for the flash column chromatography process depends
on the retention factor and it is calculated by measuring the distance covered
by the solvent as well as the sample. Some of the solvents which can be used in
the process are petroleum, Pentane, Acetate, Hexane and many more. It is a must
that all of these solvents have to be used in combination with Ether.

It is also called as medium pressure
chromatography. It differs from the other techniques as the silica gel granules
used in this technique is slightly smaller and this is the reason that the flow
of solvent is restricted. In this technique pressurized gas is also used for
driving the liquid through the stationary phase. Flash chromatography is
frequently used nowadays as it is an easy process and the equipment’s required
for this process comes in pre-packed plastic cartridges. The solvent obtained
in the process is pumped up with the help of cartridge. The unnecessary solvent
can be easily flushed out with the help of pressurized gas. Gradient pumps were introduced, and it
enhanced the separation result as now it was quicker and less solvent was used.
Flash chromatography can although not be compared with the latest High
performance liquid chromatography (HPLC), but it definitely improves the purity
of samples to quite an extent.












0 comments:
Post a Comment