The time which the compounds take to
isolate from the mixture depends on the type of absorbent used and also the
polarity of the solvent. If in case the absorbents works very fast while at the
same time the polarity of the solvent is low, then the separation process
consumes time, but the separated components are in its purest form. On the
contrary, if the absorbent does not able to work fast while the solvent has
high polarity, then the separation phase completes at a rapid speed, but the
separation quality is very poor. So in this state we can say that the separated
components are not in its purest form.
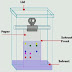
The advantage of using this technique
over the other chromatography technique is that it uses less solvent and works
faster with greater flexibility. The obtained result is obviously higher as
compared to the other processes because of the fine particles of silica gel and
aluminum oxide. This process of separating compounds is used in various fields
like medical chemistry, separation of natural products, laboratories research
and many more. In this method, the size of the absorbent particle affects the
flow of solvent through the column. Finer the particles will be, higher mesh
value it will have.













0 comments:
Post a Comment